
Get out there before it gets too hot 🙂
MycoGold https://biostim.com.au/shop/myco-gold/ and Seaweed Saver https://biostim.com.au/shop/seaweed-saver/

Get out there before it gets too hot 🙂
MycoGold https://biostim.com.au/shop/myco-gold/ and Seaweed Saver https://biostim.com.au/shop/seaweed-saver/
07 Oct, 11:30 am – 12:10 pm
Bolobek, Mount Macedon Rd, Mount Macedon VIC 3441, Australia
We’re thrilled to welcome the remarkable Keith Edwards as our guest speaker for Saturday, October 7th, at 11:30am during the Garden Lovers’ Fair.
With an incredible 40 years of experience in the landscape and gardening industry, Keith brings a wealth of knowledge to the stage. He’s worn many hats over the years – from landscape design and construction to managing the Diggers Club and overseeing massive displays at the Melbourne International Flower & Garden Show.
Keith’s passion lies in designing garden landscapes inspired by the likes of Edna Walling and Gertrude Jekyll.
His landscape plans are rooted in soil modifications and amendments, making gardens flourish.
But that’s not all – Keith is an expert in edible gardens. Most of his clients request elements of edible landscapes, with some even opting for full-fledged working edible gardens. He’s a master of raised vegetable beds that seamlessly blend function with ornamental beauty. Think espaliered fruit trees, multi-grafted wonders, and walls adorned with evergreen citrus.
Don’t miss this opportunity to gain insights from a true garden visionary. Book your tickets to the Garden Lovers’ Fair via our website.
https://www.gardenloversfair.com.au/event-details/keith-edwards-soil-soil-additives

Farmers have a golden solution to global warming largely missed by climate change pundits, right beneath their feet. The innovative Australian Soil Carbon Accreditation Scheme is showing how incentive payments can be received by landholders for measurable increases in soil carbon that soaks up CO2 from the atmosphere. Financial incentives could help fund soil restoration efforts, which in turn bring the bonus of greater productivity, drought resilience and even rain. The action is deep underground.

Australian soil scientist Dr Christine Jones is frustrated that the world hasn’t fully cottoned on to the important role of healthy crop roots and soils to draw down massive amounts of carbon, buffer drought and re-supply essential nutrients which have been drained from our landscape and farm produce by traditional agricultural practices.
Her 10-year crusade to raise the profile of soil carbon processes and what she calls the microbial ‘carbon highway’ led to the foundation of the organization Amazing Carbon and then to the development and leadership of the Australian Soil Carbon Accreditation Scheme (ASCAS).
ASCAS is a vehicle to demonstrate through farm trials that with biologically based protocols involving perennial (long-lived) deep-rooted pastures and annual crops, measured increases in vital soil carbon can be achieved quickly and rewarded with incentive payments for the CO 2 sequestered. The scheme is the first of its kind in the Southern Hemisphere, making Australia an early leader in the recognition of soils as a verifiable carbon sink.
The ASCAS project is also collecting much-needed hard data on soil carbon accumulation rates across various properties and soil types in the Northern Agricultural Region (NAR) of Western Australia and central Queensland, two of the areas hardest hit by climate change.
After the oceans, the soil is the earth’s largest carbon sink – but plants are the facilitators. Through photosynthesis plants convert CO 2 to sugars to power growth, releasing oxygen into the atmosphere. The activities of symbiotic bacteria and fungi, associated with roots and fed by the sugars, enable the exuded carbon to be combined with soil minerals and made into stable humus1 which locks the carbon away.
The fundamental processes which produce humified soil carbon are part of the microbial bridge – the focus of Dr Jones’ interest – and the key to the formation and maintenance of healthy topsoils with high moisture-holding capacity, which largely determines plant and crop productivity.
‘This can’t happen where farm chemicals kill the essential soil microbes,’ says Dr. Jones. ‘When chemical use is added to intensive cultivation, which exposes and oxidizes the humus already in the soil, it is easy to see why soil has become a huge net source rather than a net “sink” for atmospheric CO2 under current farming practices.’
Alongside this, the removal of groundcover interrupts the important water and climate cycles facilitated by plants. Photosynthesis is a cooling process. Lack of green cover on the land greatly increases heat absorption, causing a dramatic increase in evaporation. Water vapor is a greenhouse gas of greater significance for global warming than CO 2. Lower rainfall can also result from groundcover loss.
Under conventional cropping practices, soil carbon in Australia has declined to one-half to one-third of original levels. CSIRO research has found that the rate of carbon sequestration resulting from good continuous pastures is enough to maintain or increase soil carbon levels, but all other crops/pasture rotations cause a decline of surface soil carbon.

Dr. Jones claims that conventional approaches to modeling soil carbon, while useful for describing soil carbon loss, are inadequate for determining soil carbon gain. Soil carbon models such as Roth C do not take into account the humification of root exudates or contributions from mycorrhizal fungi. ‘Sequestration rates under regenerative agricultural regimes may be quite a bit higher than estimated by current models,’ she says.
The scheme is the first of its kind in the Southern Hemisphere, making Australia an early leader in the recognition of soils as a verifiable carbon sink.

Tim Wiley, Development Officer with Western Australia’s Department of Agriculture and Food, was quick to realize the great potential of soil carbon increases with perennials. Wiley has been supporting the ASCAS trials in the NAR of WA. ‘The trend is clear – perennial pastures sequester 5 to 10 tonnes of CO 2 per hectare annually.’ He says with changes to farming practice, landholders in the northern agricultural areas of WA could sequester these amounts of CO 2 over two million hectares of poor sandy soil.
‘If all WA’s agricultural soils were sequestering carbon, we would soak up WA’s current emissions. This would have the potential to significantly decrease Australia’s net emissions and meet our Kyoto obligations.’
Add in the rest of Australia’s agricultural land area – and the world’s – and the impact on global CO2 levels is evident. Wiley pointed to current cost and data limitations to quantitative measurements of soil carbon. ‘We don’t know enough about carbon under different farming systems,’ he said. ‘We have data from farmer sampling before and after perennials were planted and over-the-fence comparisons, but it is not rigorous enough.
‘To trade carbon we need a working model such as Roth C for estimating changes in carbon. The model results would be verified by occasional soil sampling of farmers’ paddocks. Roth C needs to be validated with data from long-term trials in the regions that accurately measure carbon.’ That’s where the ASCAS trials are filling in the picture.
Up at Lancelin, about 140 km north of Perth, things have been tough during the last 10 years of below-average rainfall. But cattle farmer Bob Wilson hasn’t been too fazed; since changing his farming system over the past 20 years from traditional annual pastures to the fast-growing fodder shrub, tagasaste, and subtropical perennial (permanent) grasses, his farm has ridden out the dry far better than most, producing good returns.
As a member of the Evergreen Farming Group, he hasn’t been surprised by the exciting results of the ASCAS trials. His band of farmers advocates growing hardier perennial plants which improve the soil and help stave off salinity. Two decades ago the view to the future of farming opened up for Wilson and his colleagues, and it looked much greener.
Unlike some of the other farmers involved in the ASCAS trials, Wilson has been growing perennials for some time. Using the scheme’s protocols he has been able to measure soil carbon on his land and quantify to some extent how it can improve yields, increase water and nutrient retention for greater farm vigour, and now, potentially bring useful credit income for sequestering carbon dioxide.
He now has half his 2000 ha under tagasaste in wide rows with annual pastures in between. ‘I changed my farming system because of concern for the environment, wind erosion, and our need for an extended grazing season. We have doubled our carrying capacity.’
But he points out that while subtropical perennials provide an extended period of green feed, they grow slowly during winter and are susceptible to frosts. ‘We need annuals as well for winter feed. A mixture of pasture types is best, on a case by case basis,’ he says. When Tim Wiley dug soil pits in Bob Wilson’s paddocks, he found perennial grass roots at the bottom of a 2.5 m deep pit and tagasaste roots at the bottom of a 3 m pit. He calculated from the soil test and other results that the perennial grasses and tagasaste were sequestering 7 t/ha of CO2 per year more than traditional annual
pastures.

The group of 12 farmers involved in ASCAS ‘benchmarking’ in the Northern Agricultural Region of WA over the last year will later in 2008 be completing calculations to see how much carbon has been sequestered under their perennial pastures. Baseline soil carbon levels in five increments in the 110 cm soil profile were determined during August 2007 within Defined Sequestration Areas on their properties.
Results from the first 12 months of field trials in Queensland will also be known later this year. Dr. Jones says the initial findings have been exciting. ‘One of the broadacre cropping properties north-east of Clermont in Queensland that is participating in the ASCAS project has more than three times the amount of carbon in the farmed soil than there is under the surrounding native vegetation (149 tonnes of carbon/ha under native vegetation versus 516 tonnes of carbon/ha under the crop). As a result, the soil is far more productive. The wheat crop yielded 4 tonnes per hectare of grain with 13.5 percent protein this year – well above the district average.
‘This demonstrates that with the right kind of farming (in this case zero till with microbial stimulants in place of harsh fertilizers) we can dramatically improve soil health. I’m not saying we should replace native vegetation with farmed land – far from it. What I am saying is there is still hope for much of the land that we have inadvertently almost totally destroyed,’ she observes.

Under the Australian Soil Carbon Accreditation Scheme, participating farmers will receive Soil Carbon Incentive Payments (SCIPS) calculated at one-hundredth the 100-year rate ($25 per tonne CO 2 sequestered).
The incentive payments made to farmers are a private donation from Rhonda Willson, Executive Chairman, John While Springs (S) Pte Ltd and Director, Gilgai Australia.
Receipt of Soil Carbon Incentive Payments will be similar to being paid ‘on delivery’ for livestock or grain, with the bonus being that sequestered carbon remains in the soil, conferring multiple landscape health and productivity advantages.
Agricultural soils have short-, medium, and long-term potential to mitigate climate change by sequestering atmospheric carbon as beneficial humified organic matter. Results from overseas studies indicate that the carbon sequestration potential of appropriately managed farmlands can be higher than that of tropical forests. In countries such as Brazil, Colombia, Costa Rica, Mexico, and Cuba, the science of soil carbon is the subject of active research and development.
Dr. Jones says the rationale for the ASCAS trials was to demonstrate that significant quantities of soil carbon could be sequestered on Australia’s commercial properties, even under difficult environmental conditions, provided appropriate land management technologies were employed.

Apart from needing to be rapid, stable, and applicable to large areas, soil carbon sequestration as an effective climate mitigating tool must involve low-cost, easily implemented, innovative land management techniques that differ substantially from ‘business as usual. There also needs to be effective monitoring, evaluation, and verification, particularly when measures of carbon sequestered might be linked to a financial mechanism.
At present, an emissions trading scheme does not operate in Australia, although a national scheme is planned in line with the government’s recent ratification of the Kyoto accord. Rio Tinto Coal Australia is currently one of the organizations funding research into soil carbon and its potential for a future carbon trading scheme under ASCAS.
Recent good news is that Australian agricultural products company Incitec Pivot Ltd has also come on board as a supporter of the Queensland field trials. Of the estimated 3060 gigatonnes of carbon in the terrestrial biosphere, 82 percent is in soils.2 That’s over four times the amount of carbon stored in the world’s vegetation. Dr. Jones asks, ‘If only 18 percent is stored in vegetation, why all the emphasis on biomass, rather than soil, as a carbon sink?
‘The answer is that people – including most of our top scientists – simply don’t understand soil carbon sequestration or the role of the microbial bridge and have therefore overlooked it.
‘ASCAS was established so that farmers could receive incentive payments for increases in their soil carbon. We’re demonstrating the incredible rates at which carbon can be put into soil by roots in biologically based sustainable cropping and grazing systems,’ she says.
‘Effective soil carbon management is a key factor for productive farms, revitalized catchments, and a greener planet.
‘Incentive payments for regenerative land management would help to “cash flow” the multiple natural resource management and environmental benefits that accompany increased levels of carbon in soils.’


Let’s begin with a topic that interests all farmers and one to which nearly all the other benefits of mycorrhizae are inherently linked: improving crop yields. Typically, mycorrhizae’s single most prominent contribution to a crop plant is improved access to and uptake of phosphorus (P).
All farmers are intensely familiar with the importance of this elemental nutrient to essential plant functions, which include energy transfer, photosynthesis, the transformation of carbohydrates, systemic nutrient mobilization, and genetic transfers. Given that often one of the most noticeable evidence of P deficiency in a crop is reduced yield (or in forage and pasture reduced quantity), it is no wonder that P is such a critical (and expensive) component in crop fertilizers.
Much of the naturally-occurring P in soils is found bound tightly with elements such as iron or aluminum in the form of recalcitrant compounds. Similarly, P inputs derived from fertilizers often react with ambient soil cations to form insoluble salts. In natural ecosystems, plant communities rely on mycorrhizal fungi to access these forms of phosphorus.
Mycorrhizal hyphae produce enzymes, including phosphatase to convert phosphorus into soluble, plant-usable forms. This same process can be valuable in agriculture, maximizing the availability of natural soil P as well as dramatically enhancing the efficient uptake of P derived from fertilizers. With greater P uptake, costs go down and yields frequently increase as well.
The availability of nitrogen can also be a factor in limiting crop productivity for reasons opposite to those limiting P. Available nitrogen in the forms of nitrates (N03), nitrites (N02), and ammonium (NH4) are very soluble and can flow past the root zone before roots can absorb it. This means they are often lost to run-off or groundwater or trapped in subsoil beyond the access of roots.
The profoundly dense network of tiny hyphae filaments in a mycorrhizal system typically extends 45 to 60 centimeters beyond the roots themselves, increasing the absorptive surface area of colonized roots hundreds to thousands of times. A teaspoon of mycorrhizal soil can easily contain several kilometers of hyphae, all of which are highly absorptive of soluble nitrogen ions, ensuring optimum uptake and the associated nitrogen-related cropping benefits.
Another source of nitrogen uptake unique to mycorrhizal symbiosis has recently been discovered by scientists at the University of California, Irvine, US. The researchers set out to explore how nutrients, including nitrogen, are mobilized through the environment. Using cutting-edge technology, nanometre-sized bits of a semiconducting material called quantum dots were attached to organic compounds such as nitrogen-laden amino acids.
Nutrient transfer discovery
When energised by an ultraviolet laser, the tiny dots emitted light, becoming detectable by special cameras positioned in the root zone of plants. In this manner, the scientists could track nutrients as they were absorbed into the microscopic mycorrhizal hyphae and follow their subsequent movement into the tissue of the host plant.
For more than 100 years conventional scientific wisdom held that root absorption of nitrogen was restricted to inorganic forms of nitrogen such as N03, N02, and NH4. But to their surprise, the scientists saw the illuminated dots attached to amino acids enter the mycorrhizal hyphae and observed them as entire molecules moved into the root cell vacuoles and then continued systemically to the chloroplasts (in which nitrogen is used for photosynthesis).
MYCORRHIZAE

In non-mycorrhizal rhizospheres, amino acids, which are the primary components of proteins, must undergo extensive and time-consuming decomposition processes by bacteria and other soil organisms before nitrogen is released in inorganic, plant-usable forms. In many cases, much of the nitrogen is consumed by the organisms, further delaying its plant availability.
This research demonstrates that mycorrhizal fungi allow their plant hosts to bypass this process, implementing quick and effective access to organic nitrogen sources. What this means to the farmer is that utilizing mycorrhizal fungi, naturally occurring and introduced sources of organic nitrogen (such as found in fish-based fertilizers, green manures, and compost) can provide a readily available source of nitrogen to promote crop growth and enhance yields.
In addition to phosphorus and nitrogen, the mass of hyphal filaments in the soil surrounding mycorrhizae-colonized roots is also capable of mobilizing an array of other important plant nutrients, including calcium, iron, magnesium, and critical micro-nutrients such as manganese, zinc, and copper. Just as a lack of vitamins can impair human or animal health, crop yields and forage production are sometimes limited by insufficient supplies of these minor- and micro-nutrients, even when N-P-K is abundant.
Mycorrhizae’s ubiquitous presence throughout the surrounding soil can access these relatively scarce resources and, in many cases, can release them from insoluble compounds via the production of specialized enzymes. The management of micro-nutrients is becoming increasingly recognized as an important component of modern cropping science. Mycorrhizal fungi can serve as a useful tool to ensure that both natural and introduced sources of these nutrients are transferred efficiently from the soil to the plant.

Help find water
Mycorrhizae’s significant assistance with nutrient uptake is important, but it is not the only crop-enhancing benefit offered by these amazing fungi. Another valuable feature is water management. The expanded and enormous absorptive surface area connected to the roots is going to ensure that nearly all moisture in a plant’s surrounding soil is accessed. But what then? Once the soil is dry, how can the plant survive?
Mycorrhizae provide a mechanism inside the root cells that addresses this problem. When a root cell becomes colonized by a mycorrhizal fungus, a special shared organ called a vesicle grows inside the root cell. The vesicle is essentially a storage container for water and dissolved nutrients that can be utilized in times of deficiencies, such as drought periods.
When moisture and nutrients are abundant in the soil, surplus supplies are stored in the vesicle. When moisture and/or nutrient shortages occur, the plant begins to utilize the resources stored in the vesicles to avoid stress for extended periods – often weeks or even months longer than non-mycorrhizal plants.
When moisture or nutrients again become available, the plant is able to return to normal, healthy respiration and growth without shock or other negative symptoms. Of course, the reservoir provided by the vesicle cannot last indefinitely and the plant will suffer stress and ultimately death if sufficient moisture or nutrients remain unavailable for too long.
However, in most cases the extra non-stressed time provided via the vesicle allows the plant to survive until the next rainfall. This is great news for the dryland farmer. Australia’s recent excessive rainfall notwithstanding, drought is a serious risk encountered by all dryland farmers. Although not infallible, mycorrhiza inoculation offers inexpensive crop insurance as one of its many benefits.

Introduction
There has been a notable ‘climate shift’ in many of the arable regions of eastern, southern and western Australia. A trend to less reliable autumn, winter and spring rainfall has increased production risks for annual cereal crops, while the greater incidence of episodic high intensity rainfall events in summer has heightened the vulnerability of bare fallows to erosion. Declining rainfall experienced over the last 7-10 years has severely impacted on the financial viability of cropping and grazing enterprises and disrupted the social fabric of rural communities.
These events have highlighted a fundamental lack of resilience in current agricultural production systems.
Historical losses of soil and soil carbon
In little over 200 years of European settlement, more than 70 percent of Australian agricultural land has become seriously degraded. Despite efforts to implement ‘ best practice’in soil conservation, the situation continues to deteriorate.
On average, 7 tonnes of topsoil is lost for every tonne of grain produced. This situation has worsened in recent years due to an increased incidence of erosion on unprotected topsoils, coupled with declining yields.
The most meaningful indicator for the health of the land, and the long-term wealth of a nation, is whether soil is being formed or lost. If soil is being lost, so too is the economic and ecological foundation on which production and conservation are based.
In addition to the loss of soil itself, there has been a reduction of between 50% and 80% in the organic carbon content of surface soils in Australia since European settlement (2, 3, 4, 11, 12).
Losses of carbon of this magnitude have immeasurable economic and environmental implications. Soil carbon is the prime determinant of agricultural productivity, landscape function and water quality.
Further, the carbon and water cycles are inextricably linked. Humus holds approximately four times its own weight in water (8). The most beneficial adaptation strategy for climate change would therefore be one that focuses on increasing the levels of both carbon and water in soils.
Discussions on adapting to climate change are irrelevant unless they focus on rebuilding healthy topsoil.
Building new topsoil
Healthy groundcover, active root growth and high levels of microbial association (7), are fundamental to the success of any endeavour to build new topsoil. These factors are absent from conventionally managed broadacre cropland.
Current ’best practice’, that is, chemically-based zero-till broadacre cropping (Fig.1) does not provide a suitable environment for high levels of biological nitrogen fixing, nutrient cycling, hydraulic redistribution, active sequestration of humified soil carbon, or soil building.

Fortunately, the highly effective land management technique of ‘perennial cover cropping’ (Figs. 2, 3, and 4) has become more widely adopted in recent years. This practice involves the direct drilling of annual grain or fodder crops into ‘out-of-phase’ dormant perennial groundcover.

The essential first step to rebuilding topsoil is to maximize photosynthetic capacity. A permanent cover of perennial plants provides an ongoing source of soluble carbon for the soil ecosystem, buffers soil temperatures, inhibits weeds, reduces erosion, improves porosity, enhances aggregate stability and water infiltration, slows evaporation, and ‘conditions’ the soil for the production of healthy, high quality, over-sown annual crops.
The soluble carbon exuded into the rhizosphere by perennial groundcover plants and/or transported deep into the soil by mycorrhizal fungi provides energy for the vast array of microbes and soil invertebrates that produce sticky substances enabling soil particles to be glued together into lumps (aggregates). When soil is well aggregated, the spaces (pores) between the aggregates allow the soil to breathe, as well as absorb moisture quickly when it rains. Healthy topsoil should be ‘more space than stuff’, that is, less than 50% solid materials and more than 50% spaces.
Friable, porous topsoils make it easier for plant roots to grow and for small soil invertebrates to move around. Well-structured soils retain the moisture necessary for microbial activity, nutrient cycling, and vigorous plant growth and are less prone to erosion. Soil structure is very fragile and soil aggregates are continually being broken down. An ongoing supply of energy in the form of carbon from the rhizosphere exudates of actively growing plants and, to a lesser extent, decomposing organic materials, enables soil organisms to flourish and produce adequate amounts of the sticky secretions required to maintain soil structure and function.
Healthy, chemical-free soils also create appropriate conditions for humification (conversion of soluble carbon to humus), a process that does not occur in most conventionally managed agricultural soils.

Cropping into dormant perennial groundcover is a one-pass operation that markedly reduces fuel costs and largely eliminates the need for fossil-fuel-based herbicides, fungicides, and pesticides. Perennial cover cropping has many similarities to annual cover cropping but brings with it the ecosystem benefits of perennial groundcover. The practice of perennial cover cropping was inspired by the highly innovative integrated cropping and grazing technique of ‘pasture cropping’ initiated by Darryl Cluff over a decade ago and further developed by Colin Seis (1, 5, 6).
The use of ‘biology friendly’ fertilizers, particularly those based on humic substances, in combination with Yearlong Green Farming (YGF) techniques such as perennial cover cropping, can have a protective effect on soil carbon, slowing or preventing its decomposition and further reducing the carbon footprint of agriculture.
There is no valid reason for the Australian agricultural sector to be a net emitter of CO2.
The world’s soils hold three times as much carbon as the atmosphere and over four times as much carbon as the vegetation. With 82% of terrestrial carbon in soil (compared to only 18% in vegetation), soil represents the largest carbon sink over which we have control. Soil is also the world’s largest store of terrestrial diversity, with over 95% of life forms being underground (that is, only 5% of biodiversity is above ground).
Sequestering humified carbon in soils represents a practical, permanent and productive solution to removing excess CO2 from the atmosphere. By adopting regenerative soil-building practices, it is practical, possible, and profitable for broadacre cropping and grazing enterprises to record net sequestration of carbon in the order of 25 tonnes of CO2 per tonne of product sold (after emissions accounted for).
Australia’s annual emissions of CO2 are predicted to reach 603 million tonnes in 2008.
There are therefore 603 million good reasons for agriculture to be a net sequester of CO2.
It would require only a 0.5% increase in soil carbon on 2% of agricultural land to sequester all Australia’s emissions of carbon dioxide (1). That is, the annual emissions from all industrial, urban, and transport sources could be sequestered in farmland soils if the incentive was provided to landholders for this to happen.
This would provide Australia with a 50-year window of opportunity to be carbon neutral while implementing viable technology to meet future energy needs.
Australian Soil Carbon Accreditation Scheme (ASCAS)
Dr. Christine Jones launched the Australian Soil Carbon Accreditation Scheme (ASCAS) in March 2007. ASCAS is a stand-alone incentive scheme with voluntary involvement, which encourages the adoption of innovative soil building practices (9). Widespread implementation of techniques developed by leading-edge landholders (as depicted in Figs. 2, 3, and 4) will transform the agricultural sector. Adoption of these processes needs to be fast-tracked.
ASCAS is the first incentive payments scheme for soil carbon in the Southern Hemisphere, placing Australia among world leaders in the recognition of soil as a verifiable carbon sink.
Incentive payments for annual measured increases in soil carbon above baseline levels have been sourced from a private donation by Rhonda Willson, Executive Chairman, John While Springs (S) Pte Ltd, Singapore. Receipt of Soil Carbon Incentive Payments (SCIPs) is similar to being paid ‘on delivery’ for livestock or grain, with the bonus being that sequestered carbon remains in the soil, conferring multiple landscape health and productivity advantages. Soil Carbon Incentive Payments are calculated at one-hundredth the 100-year rate ($25/tonne CO2-e).
A 0.5% increase in soil carbon across only 2% of agricultural land would sequester 685 million tonnes of CO2, well above the country’s annual emissions. (Assumptions: 0-30cm soil profile, bulk density 1.4 g/cm3, land area 2% of 445 million hectares).
Annual payments to landholders based on measured soil parameters provide an incentive for maximizing soil carbon sequestration and maintaining the permanency of sinks.
The amount of humified carbon in soil is directly related to nutrient bioavailability, soil structural stability, soil water-holding capacity, agricultural productivity, and landscape function. One of the aims of the ASCAS project is to collect data that will enable rigorous scientific evaluation of soil carbon, water, nutrients, and crop yield under regenerative regimes.
Adapting to climate change
There is an urgent need for Australian agricultural industries to adapt to climate change. To be effective, the strategies employed will require radical departures from ‘business as usual.
It is possible that global warming could accelerate even more rapidly than observed to date. Fundamental redesign of agricultural production systems will enable the sequestration of more carbon and nitrogen than is being emitted, as well as enhancing soil water retention, improving the resilience of the resource base, and restoring richness to farmed soils. These much-needed changes will assist the agricultural sector to deal confidently with a changing climate.
Rather than increase costs, mitigation of climate change via the adoption of regenerative soil building practices would bring net financial benefits to landholders and rural communities (the sectors hardest hit by climate change).

Yearlong Green Farming (YGF) techniques such as perennial cover cropping rapidly build humified soil carbon, improving the capacity of soil to hold water and increasing the resilience of farming systems to climatic extremes.
Farming in a perennial base
A change to farming in a perennial base has many advantages, including
2. fewer inputs, resulting in higher gross margins per hectare
3. less reliance on fossil fuel-based fertilizers and farm chemicals
4. enhancement of natural soil building processes
5. ‘reverse’ carbon footprint – more carbon sequestered than emitted
6. ‘reverse’ nitrogen footprint – more nitrogen fixed than emitted
7. increased water use efficiency due to lower evaporative demand
8. improved soil water balance due to hydraulic lift and hydraulic redistribution
9. no bare soil for weeds to grow – paddocks virtually weed-free
10. reduced financial risk – no expenditure if a crop is not sown
11. an additional income stream from harvest and sale of perennial grass seed
12. more time for family – little or no requirement for cultivation or herbicide application
13. higher biodiversity of plants and animals (eg bettongs returning on some farms)
14. incentive for all members of the farm family, including children, to become involved
The new face of agriculture
Widespread adoption of productive and resilient agricultural practices that enhance net sinks for atmospheric carbon would have a revitalizing effect on the natural resource base and provide a financial benefit to the government, individuals, and rural and regional communities.
Furthermore, farming in a perennial base would enhance the resilience of the agricultural landscape to a wide range of climatic extremes, some of which may not even have been encountered to date.
The development of an appropriate incentives framework for regenerative agricultural activities would reverse the farm sector’s carbon and nitrogen footprints (more C and N sequestered than emitted) and improve food security in a warming, drying environment.
An overview of the Australian Soil Carbon Accreditation Scheme (ASCAS) has been provided as an example of an incentive-based (rather than regulatory) approach. The ASCAS project is an initiative designed to provide proof of concept that: –
2. improvements in soil carbon and soil health can be measured
3. landholders can be financially rewarded for building soil carbon
The ASCAS project supports soil restoration by providing financial incentives for landholders to move away from ‘business as usual’ (that is, carbon depleting activities) and by improving community knowledge on effective methods for building soil carbon.
Irrespective of climate change, it would be of enormous economic benefit to the agricultural sector to rebuild soils by implementing practices that increase levels of humified soil carbon and reduce reliance on fossil fuels.
In 1937, Franklin Roosevelt (10) stated “The nation that destroys its soil destroys itself”. The future of Australia depends on the future of our soil.
REFERENCES CITED




Thank you all for your support in 2022 and Merry Christmas.
If you are looking to spoil yourself or a green thumb for Christmas consider some MycoGold https://biostim.com.au/shop/myco-gold/


Phosphate fertiliser prices are once again extreme. This effects super phosphate, single super, triple super, MAP, DAP, MKP, etc pricing and availability. Supply and demand determines prices but these increases are mainly on the supply side. The war in Ukraine has caused sanctions on Russia further limiting the number of suppliers in the world market. Morocco basically controls the world price as the world has under invested for decades in developing phosphate mines.
The ABC just did a video below but I have a few issues with the reporting.
1/ Australian soils have large reserves of phosphate but they are often in plant unavailable forms.
2/ Using tax player funding to build infrastructure isn’t moral or wise as the price will correct at some stage and we are a high cost producer.
3/ Rock phosphate may never become plant available. You need extensive microbial activity (labile carbon and moisture) and/or low soil pH to release the phosphate. Applying to dead soil is a total waste of resources/capital.
A more sustainable solution that requires no tax payer funding is mycorrhizal fungi as that can help unlock existing phosphate reserves. Even better than mycorrhizal fungi is implementing a cover crop strategy if possible.
Thank you to Lois D for taking the time to write a review after your purchase. We will send your MycoGold shortly.
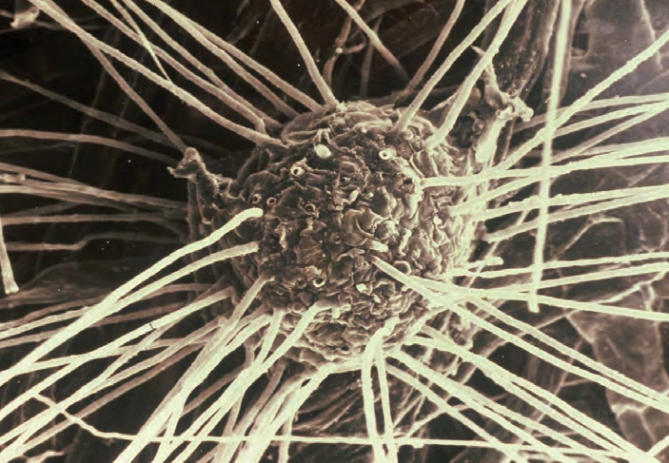

Mycorrhizae are tiny, beneficial organisms that live in the soil and connect to plant roots, providing them with moisture and nutrients. The tiny fibers are called hyphae.
Living Soil is very important to plant care. It is no surprise then that nursery professionals continue to increase their understanding of it.
Living soil includes a myriad of soil-dwelling organisms, including bacteria, fungi, soil arthropods, and a wide variety of others. One of the most intensive studies groups in recent years also has the most potential for use by nursery professionals; mycorrhizal fungi.
My-Co-Rise-ee
There is a special relationship that exists between plant roots and certain types of fungi. Which are called mycorrhizae. The name is pronounced by my-co-rise-ee. Its literal meaning is “Fungus Roots”(“myco” meaning fungus “rhiza” meaning root).
These fungi are a major component of a multitude of hardworking armies of beneficial soil organisms largely invisible to us beneath the soil surface.
The mycorrhizal relationship is a symbiotic relationship. Both the plant and the fungus benefit. Nearly all horticulturally important plants and approximately 90 percent of all higher plants depend on the mycorrhizal relationships in their natural habitats.

MYCORRHIZAE
These hard-working fungi provide the cornerstone for sustainability of our plant communities. They provide the moisture and nutrients needed to keep plants in our natural areas healthy and functioning through tiny absorptive threads called hyphae.
We could not survive a day without them. Without their diligent munching in the soil, plants in native ecosystems all over the world would go hungry and die of thirst.
Ancient workers
Since the early days, 460 million years ago, these mycorrhizal fungi have been amazingly prolific. Miles of fungal filaments can explore a single thimbleful of healthy soil. They pluck phosphorus, nitrogen and micronutrients out of the soil with a specific arsenal of designer enzymes just right for the job.
Mycorrhizal fungi process waste and make it usable again, purify our water, and keep our plant communities productive. The wide variety of nursery plants will thrive when given the right source of mycorrhizal inoculum in areas where it has been lost to disturbance or not present in sterile soil mixes.
Mycorrhizal fungi attach themselves to the roots of plants and radiate out into the soil, helping their host plants absorb water and nutrients. In return, the host tree feeds the fungi with sugars, proteins, amino acids and other organic substances.
Fungi are made up of filaments called hyphae. A mass of hyphae is a mycelium, which can grow very rapidly. A fungus colony can produce more than a kilometer of new mycelium in 24 hours!
This growth form has a very high surface area. This is one of the attributes that makes the symbiotic relationship so successful. Mycorrhizae can spread their net of hyphae far and wide in the soil, penetrating tiny spaces in the soil where plant roots can’t go.
In addition, fungi are also capable of breaking down, or converting, some nutrients such as nitrogen and phosphorus to forms usable by plants.
The good news and the bad news
The good news is when water and soluble nutrients are amply provided, non-mycorrhizal plants can grow well under nursery conditions. However, until they form mycorrhizae, they don’t efficiently take up water and nutrients at the nursery or upon being planted in the ground.
Routine nursery practices such as fumigation, sterile soilless growing media and chemical use produce non-mycorrhizal plants. The bad news is that target plants do not utilize much of the fertilizer used in the nursery industry because the root/mycorrhizal system is underdeveloped.
In addition, many nursery-grown plants (and their roots) are adapted to nursery conditions and not to the highly disturbed and sometimes hostile environment found in many urban and suburban settings. In these settings, the chance of a beneficial mycorrhizal fungus colonizing the roots can be low because there may be no source of inoculum readily available.
To confirm the effectiveness and benefits of mycorrhizal treatment, I conducted a test of a mycorrhizal inoculant for four important horticultural species at Village Nursery in Sacramento, Calif.
My hypothesis was that mycorrhizal fungi could be established under nursery conditions and would increase the plants’ root system capacity to effectively uptake nutrients at levels considered by conventional standards to be lower than optimum rates. I wanted to test whether inoculated plants’ growth and development would be adversely affected as a result of reduced fertilizer inputs.
The experiment
Four plant families were tested because of their popularity in the landscape industry: 1) Cotoneaster apiculata, 2) Trachelospermum jasminiodes, 3) Pinosponim uariegate, and 4) Escallonia fradesii. The experiment had three fertilizer treatments.
1) Grower standard practice (GSP). For this control group, I applied 8 pounds Apex 23-6-12 per cubic yard (equivalent to 0.23 pounds nitrogen per cubic yard over an 8 month period). Because this was the control group, there was no mycorrhizal inoculation.
2) Apex mixed at 20 percent less than GSP. I added a fertilizer ratio of 6.5 pounds Apex 23-6-12 per cubic yard (equivalent to 0.19 pounds nitrogen per cubic yard over an 8 month period) with mycorrhizal inoculation.
3) Apex mixed at 30 percent less than GSP. I fertilized with 5.5 pounds 23-6-12 per cubic yard (equivalent to 0.15 pounds nitrogen per cubic yard over an 8 month period) with mycorrhizal inoculation.
How the experiment was conducted
For each plant species and fertilizer/mycorrhizal treatment there were 50 replications. Mycorrhizal inoculum was watered in (drenched), until water began dripping from the bottom of the 2-inch liner pots. Mycorrhizal inoculum was used at a rate of 1 pound per 200 gallons of water. Each pound treated approximately 2,000 square feet of nursery plants.
The standard fertilization (GSP) rate was not inoculated. The plots with Apex mixed at 20 percent below standard and 30 percent below standard were inoculated with Mycorrhizal inoculum. For all treatments, lime was added to the soil at the standard 7 pounds per cubic yard of soil. A premix containing other nutrients was added. It included 1 pound Nitroform fertilizer, 1 pound FeSO4 (iron sulfate) , 0.75 pounds Tiger-90 sulfur fertilizer, and 1 pound triple phosphate (fertilizing supplemental blend) per cubic yard.

All plants were allowed to continue growing for 90 days. At the end of 90 days, root systems were sampled, cleared, and stained to determine the presence of mycorrhizal colonization of the plant root systems. Afterward, plants were transplanted as 2-inch liner pots into 1-gallon containers. Plants were set up aside the GSP in the grow grounds under the typical Rain Bird irrigation system. These plants were monitored for visual differences in growth and development. Random subsamples of Rscallonia Weir were selected for biomass measurements.
Results
Mycorrhizal colonization averaged 48 percent and 56 percent for the Mycorrhizal inoculum treatments with fertilization reductions of 20 percent and 30 percent. In the untreated, or control plants, there was only 3 percent mycorrhizal root colonization. No significant visual differences were detected in plant growth development between standard growing practices and 20 and 30 percent reduction in fertilizer with Mycorrhizal inoculation. In fact, in nearly all cases, plants are grown with fertilizer reduction treatments with mycorrhizal inoculation looked as good or better than the GSP.
A subsampling of Rscallonia species biomass indicated that the plants treated with 20 percent less fertilizer had 15 percent greater biomass than plants receiving the GSP treatment.
Conclusions
Mycorrhizal inoculants are not a silver bullet but are another valuable tool available to the nursery professional. Mycorrhizal colonization was achieved by a simple inoculum drenching of the plant material. In this experiment, a significant reduction of fertilizer inputs accompanied by mycorrhizal inoculation of a plant’s root system achieved a high level of mycorrhizal colonization. The plants that received mycorrhizal inoculations and were treated with 20 percent or 30 percent less fertilizer than standard practice did not suffer adverse plant growth or development.
Establishing nursery plants on disturbed sites requires an understanding of the many soil processes important in facilitating uptake, storage, and cycling of nutrients and water. In natural areas, these activities are largely performed by a diversity of beneficial soil organisms. These include mycorrhizal fungi working hard below the living soil surface.
In past decades, clearing of natural areas, compaction, and disturbances in suburban and urban environments have substantially reduced mycorrhizal populations. Reestablishing these beneficial fungi can occur at the nursery.
The result can be substantial fertilizer savings without adversely affecting plant growth and development. The resulting will have root and mycorrhizal systems that are well suited for the out-planted environment.
Mycorrhizal fungi gets a mention